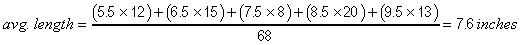
1. Lakes
The LAKE PHYSICAL DESCRIPTION form is to be used to record observations of the watershed and the lake basin. Comments on the drainage should note potential problem areas requiring frequent observation. These would include areas of potential erosion, contamination or alteration. Sources of contamination should be brought to the attention of Department of Natural Resources enforcement personnel.
Several lake basin measurements (area, depth) can be taken from topographic maps, while others (flushing rate) must be calculated in the office and may not be determined until needed. Heating degree days is required mostly for research purposes, and will be recorded by research personnel from the literature cited. All other information requested on the LAKE-PHYSICAL DESCRIPTION form should be completed.
Photographs of potential problem
areas are valuable historical evidence, and can be filed with
the report.
2. Streams
The STREAM SURVEY SUMMARY form will be used to record characteristics of streams and their watersheds. Even though the form is designed to describe a stream, most of the recorded information will by necessity reflect study stations. A complete stream description will thus consist of the summation of data from several, or many, stations.
Conditions on streams or their watersheds which are creating (or may create) management problems should be recorded. These include such things as: (1) erosion from stream banks, roads, timber cutting operations, development, etc.; (2) impoundments made by man or beaver, outflows from ponds dredged adjacent to streams, (3) barriers such as dams, culverts, waterfalls, etc.; and(4) pollution which might involve chemical toxicants in the stream and/or aquifer, commercial fertilizers, sewer effluents (and seepage), sedimentation, temperature degradation, etc.
The quality of streams as fish habitat is largely determined by the relative size, depth and frequency of pools. In general, good pools are deeper and wider than the average width and depth of the stream. Current must be reduced and cover should be present in order to constitute good fish habitat.
Pools should be judged by their size, type and frequency. The following classification is from Lagler's (1952) "Freshwater Fishery Biology" (W. C. Brown Co., Dubuque):
Size
1. Large: Pools having an average width greater than the average width of the stream.
2. Average: Pools having a width equal to the average width of the stream.
3. Small: Pools narrower than the average stream width.
Type
1. Deep: Pools exceeding 2 feet deep; exposed pools with luxuriant aquatic plants harboring a rich fauna; or deep pools with abundant cover of logs, roots, boulders or overhanging bank, much drift or detritus, and shaded by bank vegetation.
2. Moderate: Pools intermediate in depth, shelter and plant abundance.
3. Shallow: Shallow exposed pools without cover and without plants; scouring basins.
Frequency
1. Many: More or less continuous pools; ratio of pools to riffles about 75% to 25%.
2. Frequent: Rather close succession of pools and riffles in approximately a 50% to 50% ratio.
3. Infrequent: Long stretches
of shallow riffles between pools; pools making up less than 25%
of the entire stream area.
All streams have been classified by the Michigan Stream Classification System (VI-A15), and the classification should be listed on the STREAM SURVEY SUMMARY form. Streams are classified by the following system:
Top Quality Trout Mainstream.-Contain good self-sustaining trout or salmon populations and are readily fishable, typically over 15 feet wide.
Top Quality Trout Feeder Stream.-Contain good selfsustaining trout or salmon populations, but difficult to fish due to small size, typically less than 15 feet wide.
Second Quality Trout Mainstream.-Contain significant trout or salmon populations, but these populations are appreciably limited by such factors as inadequate natural reproduction, competition, siltation, or pollution. Readily fishable, typically over 15 feet wide.
Second Quality Trout Feeder Stream.-Contain significant trout or salmon populations, but these populations are appreciably limited by such factors as inadequate natural reproduction, competition, siltation, or pollution. Difficult to fish because of small size, typically less than 15 feet wide .
Top Quality Warmwater Mainstream.-Contain good selfsustaining populations of warmwater game fish and are readily fishable, typically over 15 feet wide.
Top Quality Warmwater Feeder Stream.-Contain good selfsustaining populations of warmwater game fish, but are difficult to fish because of small size, typically less than 15 feet wide.
Second Quality Warmwater Mainstream.-Contain significant populations of warmwater fish, but game fish populations are appreciably limited by such factors as pollution, competition, or inadequate natural reproduction. Readily fishable, typically over 15 feet wide.
Second Quality Warmwater Feeder Stream.-Contain significant populations of warmwater fish, but game fish populations are appreciably limited by such factors as pollution, competition, or inadequate natural reproduction. Difficult to fish because of small size, typically less than 15 feet wide.
Streams, or stream sections, which currently receive significant runs of anadromous trout or salmon are also to be designated as trout streams, regardless of whether they are d trout' or "warmwater" according to the above classification.
For a broader overview of the drainage characteristics, a narrative should be written describing the soils, topography, vegetation classification, land use, unique features, and problems. When more detail is desired and, to provide a better conceptual picture of the drainage, a topographic map may be prepared showing its principal features.
Streams are described by establishing habitat inventory sites which may be divided into zones and stations.
a. Zones.-First, partition the stream into segments (zones) about 8 km long. This can be done on drainage topographic maps. If you want to number these zones, start at the stream mouth and number consecutively as you proceed up the mainstream to its source. Then number the tributary zones similarly beginning with the lowest tributary in the drainage (Fig. II-1).
b. Stations.-The station is the basic sampling unit where most measurements of the stream's physical, chemical, and biological parameters will be made. Select one (or more if necessary) sampling station near the center of each zone. The station must be representative of its zone and should be easily located from landmarks.
c. Length.-A sketch of the sampling station should be made on the Field Map Sheet which is available for field use (Fig. II2). The sketch should indicate directional orientation and note prominent features of the landscape (roads, bridges, etc. ). The length of the station is measured down the center of the stream, and stream width is measured at 25-meter intervals. Determinations of average stream width and station area can be made on the Field Map sheet. The length of the station can vary depending on density of the fish to be censused and your efficiency in capturing them. A 400-meter station is usually adequate for trout in northern lower peninsula streams. However, it appears that a length of 800 m may be required for trout in upper peninsula streams, because these streams generally have lower trout densities and lower electrofishing efficiency (due to lower conductivity). As a rule of thumb, for determining the length of a sampling station, electrofish until at least five fish in each size class common to the population have been captured. Electrofishing for trout is used here as an example but the rule applies for other target species and sampling gear. It is best to have the station terminate at a 50-m interval to minimize problems of calculation. Record these length intervals as in Table II-1. Both the upper and lower boundaries of the station should be permanently marked. Best markers are metal stakes placed at boundaries or pins driven into witness trees near boundaries. Describe the location of markers in field notes.
d. Width.-Take width measurements at each 25-m interval as you progress downstream. Width is measured from water's edge (left bank) to water's edge (right bank) at a right angle to the bank. Record width as in Table II-1. Area can be calculated by multiplying average width times the station length. When an island occurs in the stream, width measurements should be taken across the stream including the width of the island (Fig. II-3). Then subtract the area of islands to arrive at the water area only. A fairly accurate estimate of most islands in streams can be made with the following formula: island length X maximum width of island X 0.6. If the island is not of typical form (teardrop), then an array of width measurements should be taken. Area of the island is then calculated by multiplying the average width times length. Note that in the future we may wish to quantify certain measures of a fish population and express them in terms of the static water volume of a stream, its volume of flow per unit time, or even its total annual flow. These expressions may have a better biological basis for streams than the ones used at present -- fish per unit length or fish per unit area.
e. Depth.-Measure depth at 0. 5-m intervals (0. 25 m, 0. 75 m, 1. 25 m., etc. ) along the stream width cross sections. Record depth measurements as on Table II-1. Measure from the water surface to the top of the substrate. Be careful not to disturb the top of soft bottom sediments .
f. Cross section profiles.-Cross section profiles graphically indicate the quality of stream fish habitat, since a summation of stream profiles indicates morphological diversity of the stream channel. Good stream habitat consists of a diverse blend of pools and riffles. Profiles can be drawn and their area calculated from each set of width and depth measurements. To calculate area, multiply the width times the average depth at each particular cross section. These profiles can be used to calculate the static water volume of the study station.
g. Static water volume.-This parameter has considerable biological significance because it is the total potential living space available for fish. To calculate the static water volume within the sample section, first determine the average cross sectional profile area. The average profile area times the section length equals static water volume. This approach eliminates problems caused when islands occur within the sample station. Do not calculate the static water volume by multiplying the average depth of the cross sections times the average width times the sample section length. This procedure gives an overestimate of water volume .
h. Discharge.-The best place to measure stream discharge in the sampling station is where the stream channel is straight and canal-like. The more laminar the water flow, the better the velocity measurements will be. Discharge measurements should be made using standard procedures with a Gurley current meter (VI14). Note, since the meters available at present are calibrated in English units, discharge will have to be calculated in these units, then transformed to metric units (m3/sec). The best time of the year to measure discharge for our purpose is during October or November because the streams are generally in their most stable flow conditions and near their average seasonal flow. Take measurements 3 or 4 days after the last precipitation.
i. Velocity.-Average stream velocity can be calculated by dividing discharge by average cross sectional area. Velocity is highly variable within a cross section, between cross sections within the stream reach, and at different stream stages or discharges.
j. Annual stream discharge.-In the future we may want quantitative measurements of populations in terms of numbers, biomass, or production per total annual volume of flow. To obtain the annual discharge for a stream, it is best to have a continuous recording of the water height (stream stage). This, along with discharge measurements at an array of stream stages, provides the means to construct a rating curve from which the annual discharge can be calculated. A second method is to calculate annual discharge from known monthly flow periodicity. A third method, that is less precise but satisfactory for our purpose, is to assume that discharge (in m3 /sec or c. f. s. ) during October or November equals the average discharge during the year and multiply it by 31, 557, 600 (the number of seconds in a year).
k. Stream stage.-Stream stage is the relative change in water surface height as measured on a staff gauge. It is best to record this continuously with an automatic recorder. Next best is to read it daily or periodically. As mentioned earlier, if the stream stage is known, and there is a stream discharge rating curve for various stream stages, the total river flow can be determined.
1. Gradient.-Stream gradient, expressed as drop in elevation per kilometer or percent slope, can be estimated from contour lines of U. S. G. S. topographic maps. More precise measurements would require the use of surveying instruments (transects or dumpy levels along with measurement of drop below the line of sight).
m. Bed type.-Streambed refers to the veneer of sediments at the earth-water interface. Bed types should be recorded when depth measurements are taken. These records can then be summarized as percentage sand, gravel, clay, detritus, etc., for the entire stream. Another way of measuring bed type or composition is to take scoop samples along the line transects with appropriate sampling apparatus, then sieving the samples through standard Tyler sieves to determine the size distribution of the particles.
n. Spawning areas.-In the past many surveys have attempted to assess spawning areas for salmonids based upon the percent gravel in the streambed. There are reservations as to the value of this approach because not all gravels are used by fish. Use depends upon factors such as groundwater upwelling, temperature, dissolved oxygen, bed porosity, bed permeability, and the salmonid species and their size. A more accurate assessment of spawning habitat can be made by walking or canoeing the stream during the spawning period and noting where redd building activity and spawning actually occurs .
o. Cover.-Cover
can be in the form of logs, brush, rocks, turbulent water, turbid
water, water depth, undercut banks, or objects hanging over the
water--anything providing shelter for fish. Cover is highly variable,
and its characteristics are not readily quantified. Subjective
terms such as "good", 'moderate ', or "poor"
are usually adequate for stream inventories.
1. Lakes
Routine limnological measurements will be made and recorded on the LIMNOLOGY form. Two levels of intensity will be employed in limnological lake surveys, depending on the scope of other biological studies being conducted. A first level survey will be associated with routine fish collections or other sampling, short of a complete biological survey. A survey at this level will mostly be restricted to the measurement of parameters that will assist in fish sampling.
a. First level survey.-Measurements to be made in a first level survey include dissolved oxygen and temperature, depth profiles, alkalinity, Secchi disk, observations of water color, and influential weather conditions. Alkalinity measurements might be omitted if reliable data have been collected within the past 5 years indicating that the lake has a total alkalinity in excess of 80 ppm. Soft water lakes should be monitored at every convenient opportunity due to their lack of buffering capacity and consequent susceptibility to degradation by such phenomena as acid precipitation.
Temperature and oxygen depth profiles should be determined prior to fish sampling with any type of nets if the lake is stratified. Knowledge of these factors can prevent much wasted effort from fishing depth strata unsuitable for the target species of fish. A depth sounder should be used while setting nets, and the depth at each end of the net is to be recorded. The temperature range and the dissolved oxygen concentration within the strata fished can then be determined from temperature and oxygen depth profiles.
Complete temperature and oxygen depth profiles are not always necessary when netting during spring and fall circulation periods. However, sufficient temperature measurements must be made to assure that the lake is in a state of complete circulation. If circulation is not complete, anoxia may persist in the bottom strata.
Water transparency and color are valuable observations since they reflect the magnitude of plankton production. The Secchi disk is possibly our best available indicator of the basic productivity of a lake.
b. Second level survey.-A second level limnological study is to accompany a complete survey of a lake. In addition to the first level measurements, a second level study will include on-site observations of abundance of aquatic vegetation and the detection of pollution, or other water quality problems, which may need more study by the Water Quality Division. Water samples will also be collected and sent to the Environmental Services Laboratory for extensive chemical analysis. The results from these analyses will be incorporated in the data storage bank of the Inland Lake Management Unit of the Land Resource Programs Division as part of their intensive lake surveys. These data will be stored in STORET, but will also be available for our files. Sample and field information requirements are contained in VI-16.
The Environmental Services Laboratory will analyze the following parameters for all lake surveys:
| pH | Total phosphorus |
| Total alkalinity | Soluble ortho-phosphorus |
| Conductivity | Nitrate and Nitrite |
| Chlorides | Ammonia |
| Suspended solids | Organic nitrogen |
| Total solids |
The following parameters may be measured also the first time a lake issurveyed. These will include:
| Hardness | Total iron |
| Turbidity | Magnesium |
| Silica | Potassium |
| Calcium | Sodium |
| Sulfate | Total organic carbon |
It is essential that both on-site
measurements and collection of water for laboratory analysis take
place during the time of maximum summer stratification--mid-July
to mid-September. This is the only time that we can determine
the maximum extent of oxygen depletion in the hypolimnion, and
consequently, the suitability of the lake for cold water fish.
It is essential that a schedule of lakes to be included in the
intensive surveys be sent to the Inland Lakes Management Unit
during the December prior to the surveys. This enables the laboratory
to schedule the analyses required. Laboratory services are allotted
in January for the entire year.
2. Streams
a. Temperature.-A common procedure is to record air and water temperature and time of day at each survey outing. This meager information is of little value. Since seasonal and daily fluctuations in temperature are among the most important environmental factors affecting fish, we should make an effort to obtain good temperature data. Maximum-minimum thermometers should be placed at various locations along the stream drainage, including major tributaries. They should be read weekly for one full year, or for at least one summer. One year of data will usually provide a good picture of the temperature regimes within the stream drainage. Salmonids have highest populations in streams with the least amount of variation in seasonal and daily temperatures. Also these are the streams with the lowest average annual water temperature, particularly low average summer water temperatures. Undoubtedly, warmwater fish species also benefit greatly from relatively stable water temperature regimes, but, of course, on the warmwater side of the temperature scale.
b. Water chemistry.-Water
analysis for dissolved oxygen, alkalinity, and pH are recommended
for streams, for they are key indicators of the general quality
of the environment. More intensive and varied chemical analysis
should be done if pollution or some abnormal condition is suspected.
For example, large daily fluctuations in the D. O. point up pollution
problems. Many other chemical determinations, such as hardness,
total solids, phosphorus, nitrogen, etc., might be of interest,
but are too expensive for general surveys.
3. Limnological methods
a. Temperature.-In lakes, water temperature measurements should be made in øC with an electronic thermometer. A temperature reading should be taken, and recorded, at every meter of depth with the exception of the following conditions:
1. If, within the epilimnion or hypolimnion, there is no change from the reading of the previous depth.
2. If, during the spring or fall overturns, temperature is uniform with depth.
The electronic thermometer should be standardized with a good laboratory thermometer at least once per year.
In streams, or at lake surfaces, temperatures can be taken with a pocket thermometer. However, a pocket thermometer should not be used to record the temperature of a water sample that has been collected with a Kemmerer sampler and emptied into a glass bottle. Water is appreciably warmed as it is lifted through the epilimnion and emptied into a bottle. Temperatures taken in this manner can be in error by as much as 5 degrees.
When taking air temperature, be sure the thermometer is dry and shaded from the direct rays of the sun.
b. Dissolved oxygen.-Oxygen determinations must be made at sufficient depth intervals to accurately delineate stratification within the lake. Temperature stratification should be determined prior to conducting oxygen analysis. Samples for oxygen analysis should then be collected at the surface, top, middle, and bottom of the thermocline, middle of the hypolimnion, and within 1 m of the bottom. These samples should be analyzed on the lake, and then additional samples taken to describe oxygen depletion. You should look also for an oxygen maximum in the thermocline, since this is an indication of high phytoplankton abundance
If oxygen samples cannot be titrated on the lake, then additional samples must be taken initially. Samples should then be collected at the surface and bottom of the epilimion, and every 2 m of depth from the top of the thermocline to the bottom of the lake.
The oxygen content of water can be measured either by an oxygen probe and meter or by chemical analysis. An oxygen meter is advantageous when a large series of samples is to be run frequently. However, infrequent analysis of a few samples can be done almost as conveniently by chemical methods. An oxygen meter must be standardized in a water sample previously analyzed by a chemical method. Standardization must be repeated daily. Thus a few samples can be run chemically almost as fast as a meter can be standardized.
The Winkler method of chemical analysis will be used. Several modifications of this method have been advocated for waters containing various interfering substances. However, these substances are sufficiently rare in unpolluted natural water that we will use the unmodified method. Water is collected from a desired depth with a Kemmerer water sampler, and transferred to a 250-ml BOD bottle by inserting the tube of the sampler to the bottom of the bottle. Care must be taken to flush the bottle about two times its volume and not to retain air bubbles when inserting the ground glass stoppers.
1. Fixing: Three reagents are added to the sample with automatic pipets, as follows:
a. 2 ml manganous sulfate (MnSO4); deliver below the surface of the water so as not to introduce air bubbles.
b. 2 ml alkaline-iodide solution (potassium or sodium; KI-KOH or Na-KOH); add immediately following the MnSO4 . Deliver below the surface as before .
c. Replace stopper and mix thoroughly by inverting bottle repeatedly. Allow precipitate to settle until top half of bottle is clear.
d. 2 ml concentrated sulfuric acid (H2SO4); deliver carefully below the surface of the sample. Restopper and shake until precipitate dissolves. If precipitate does not dissolve immediately, allow to stand for several minutes.
2. Titrating: The sample is now ready to titrate with 0. 025 N sodium thiosulfate (Na2S203) for final analysis. Titration may be done immediately in the field, or samples may bereturned to the lab and held for several days. If necessaryto delay titration, store samples in the dark. The titrationprocedure is as follows:
a. Transfer 200 ml of sample to a 250-ml Erlenmeyer flask.
b. Titrate with Na2S203 until pale yellow color.
c. Add a "pinch" of Thyodene (starch substitute) for pale blue color.
d. Continue titration until colorless. The number of ml of Na2S203 used in the total titration is numerically equal to the dissolved oxygen concentration in parts per million (ppm or mg/liter).
3. Reagents: The reagents used in the Winkler method of oxygen analysis are prepared as follows:
Manganous sulfate solution: Dissolve 480 g MnSO4 .
4 H2O or 400 g MnSO4 . 2 H2O or 364 g MnSO4 . H2O in distilled water, filter and dilute to 1 liter
Alkaline-iodide reagent: Dissolve 500 g sodium hydroxide (NaOH) or 700 g potassium hydroxide (KOH), and 135 g sodium iodide (NaI), or 150 g potassium iodide (KI), in distilled water and dilute to 1 liter.
Sulfuric acid: Purchase concentrated solution.
Sodium thiosulfate: Purchase Acculute brand (Anachemia Chemicals Ltd., P.O. Box 87, Champlain, New York 12919) of standard volumetric solution. This comes in a small bottle which is emptied into a 1liter volumetric flask. The bottle is filled with distilled water and emptied into the flask three times, to assure complete rinsing, and the flask is then filled with distilled water. The liter of solution will be exactly 0.025 N, and will not need to be standardized as required in the past. The solution will keep for at least 6 months if refrigerated.
Thyodene:
Purchase (Fisher Scientific Co.) and use as supplied.
c. Alkalinity.-Samples should be collected from the surface,middle of the thermocline, and within 1 m of the bottom. Phenolphthaleinand methyl orange, or total alkalinity, are to be determined by the chemicalmethod, as follows:
1. Water is collected with a Kemmerer sampler, and 100 ml is transferred to an Erlenmeyer flask.
2. Add 4-5 drops of pH-th indicator. If the sample remains clear, record 0.0 pH-th alkalinity. If the sample becomes pink, titrate with 0.02 N sulfuric acid until clear. Ten times the number of ml of acid used equals the pH-th alkalinity.
3. To the same sample add 3-5 drops M. O. indicator, and, without refilling buret, continue titration until yellow color changes to salmon pink. Record total alkalinity (M. O. alkalinity) as 10 times the total number of ml H2SO4 used in both titrations.
4. Reagents: The reagents used in the alkalinity determination are prepared as follows:
Phenolphthalein (pH-th) indicator: Dissolve 5 g phenolphthalein in 500 ml of isopropyl alcohol and add 500 ml distilled water. If necessary, add 0. 02 N sodium hydroxide (NaOH) dropwise until faint pink color appears.
Methyl orange indicator solution: Dissolve 500 mg methyl orange powder in distilled water and dilute to 1 liter.
Sulfuric acid, 0.02 N:
Purchase Acculute solution and dilute to 1 liter. See instructions
for sodium thiosulfate in dissolved oxygen methods.
d. Secchi disk depth.-The transparency of water is measured by determining the depth at which a Secchi disk disappears from view when lowered through the water column. A Secchi disk is a metal plate 20 cm in diameter, with the face divided into four quadrants. Two opposite quadrants are painted black and the other two are white. A graduated line is fastened to an eye bolt in the center of the disc. Standard conditions for the use of a Secchi disk are as follows: bright day, sun directly overhead; shaded, protected side of the boat; without polarizing sunglasses. The Secchi disk is lowered into the water, noting the depth at which it disappears, than lifted, noting the depth at which it reappears. The average of the two readings is recorded as the Secchi disk depth or limit of visibility. The depth should be recorded to the nearest 0.1 m.
e. Color.-Michigan waters are either colorless (lakes may appear to be blue or green) or stained brown by humic acid from organic drainage. Color will be recorded as either clear, light brown, brown, dark brown, or turbid. Color may be determined by examination of a sample in a bottle, or as observed against the Secchi disk held a few centimeters beneath the surface.
f. Environmental Services Laboratory analysis. -Water samples for laboratory analysis must be received at the lab within 48 hours, and must be kept cold until delivered. From most areas of the state, this can be accomplished by either DNR aircraft or commercial bus . Fisheries Division personnel will pick up samples at the Lansing airport or bus depot if arranged by telephone. The Inland Lake Management Unit will furnish station location sheets and three laboratory analysis sheets. These forms should accompany the samples to the laboratory. Detailed instructions for handling samples and forms are contained in Appendix VI-A-16. Samples for nutrient analysis should be collected at the surface, mid-depth (thermocline area if one exists), and within 1 meter of the bottom. Three 500-ml plastic bottles of water from each depth are required. One bottle from each depth is to be preserved as directed
g. pH-Despite
the fact that biologists have been recording the pH of water for
many years, there still seems to be no satisfactory method of
field measurement. Portable pH meters are the preferred method
if one is available that proves to be reliable. If a meter is
not available, a HACH kit should be used. Most municipal sewage
treatment plants will do pH analysis upon request.
1. Lakes
a. Macrophytes. -Abundance of littoral vegetation is to be recorded on the LIMNOLOGY form. Abundance estimates are to be made for various forms of aquatic plants including submergent, emergent, floating, and Chara.
Aquatic plants are good indicators of lake eutrophy. Traditionally biologists have made a single statement evaluation of macrophyte abundance throughout an entire water body. Plant abundance has the potential of giving us more information than we have utilized if we can be more precise in recording our observations. This may prove to be one of our most significant historical observations for evaluating cultural eutrophication.
The recorded observations for each form of vegetation should consist of one or more percentage figures representing the percent of the littoral area where that growth form is common (C), abundant (A), excessive (E), etc. For example, if emergent vegetation is sparse in 60% of the littoral, common in 20% and excessive in 10% the recorded notation should read: Emergent 60 S, 30 C, 10 E. The recorded percentages should always total 100% of the littoral.
b. Chlorophyll-Chlorophyll analysis is the easiest and most practical method of recording phytoplankton abundance. This is also a useful historical measure of eutrophication.
Chlorophyll analysis will be conducted by the Inland Lake Management Unit. These samples must be scheduled in advance of collection.
Chlorophyll requires special collection and handling techniques. A special composite sampler (Fig. II-4) is used to collect a composite sample throughout the water column from the surface to a depth of twice the Secchi disk transparency. The sample is placed in a 250-ml dark bottle, and one drop of magnesium carbonate is added as a preservative.
c Fish food. -The sampling of zooplankton and benthos is a . time consuming task and is not recommended for routine lake surveys. However, sampling for large zooplankters, as described in Appendix VI-Al3, is recommended for special surveys of lakes in which (1) stocked trout are not providing satisfactory returns and (2) survival of walleye or other young game fish is poor.
For routine surveys, simply make
observations on fish food organisms while conducting other parts
of the survey. Watch for zooplankton blooms, insect hatches, burrowing
mayflies (or their burrows), crayfish, and forage fish. Report
noteworthy observations on the LAKE SURVEY SUMMARY form or on
a NOTES AND REFERENCE form.
2. Streams
a. Vegetation. --To assess the standing crop (or production) of plants growing in streams is extremely difficult. For most surveys, the best we can afford to do is to subjectively estimate the percent of the channel in which vegetation is "abundant", "moderate", or "sparse".
The type, size class (height), and degree of shading provided by vegetation adjacent to the stream should be noted also. For example, canary grass that overhangs a stream bank or dense tag alder (up to 12 feet high) that form a dense canopy over the stream.
b. Fish food.-An estimate of the relative abundance of fish food can be made from two square-food samples of bottom fauna-one from the middle of the stream and one midway between the center and a stream bank. Take the samples with a Surber Sampler, or a similar device, and calculate the average number and volume of organisms. The resulting estimates, based on only two samples, will be quite rough, but much more extensive sampling is required for good quantitative estimates of abundance of benthos.
Use the mean numbers and volume (or weight) of fauna from the two square-foot samples to classify the stream for food richness as follows:
Exceptional richness: Volume greater than 2 ml, or 2 g, and number of organisms greater than 50.
Average richness: Volume from 1 to 2 ml, or 1 to 2 g, more than 50 organisms.
Poor richness: Volume of benthos less than 1 ml, or 1 g, and (or) fewer than 50 organisms.
In order to qualify in any richness
category both the numerical and weight or volume requirements
must be met by the mean squarefoot sample.
1. Discussion
Samples of fish may be desired for studies at one, or all, of three levels: (a) community (species diversity and relative abundance of species), (b) population (abundance, distribution, length-frequency, age frequency, growth, etc. of a species population), or (c) individual (specimens). The sampling of communities and populations will be emphasized in the following discussion because it is essential to fisheries management and the most difficult part of fish surveys.
It is difficult to obtain a completely unbiased sample of fish living in natural habitats. Catches are nearly always affected by at least three factors: (a) gear selectivity (influencing species caught, relative abundance, size distribution, and sometimes whether the more active or the more passive individuals are captured), (b) differences in gear efficiency among habitats (e.g., most types of gear sample the shallow littoral zone most effectively), and (c) daily and seasonal changes in the behavior of fish which alter their vulnerability to capture. In addition, care must be exercised to avoid further bias when the catch is subsampled for length-frequency, age and growth, survival rate, etc.
Usually, our aim in field surveys is to obtain a representative sample of the species and sizes of interest. Unless our interests are very narrow (i.e., targeted), a variety of gear types, habitat types, sample sites, and sample dates will be required for a good representative sample.
Within this context, fish sampling should provide:
a. Enough fish of the right species and sizes to be statistically meaningful.
b An orderly and reliable information and data base.
c. A means of systematically identifying change.
d. The specific information needed to solve a specific problem.
The objective(s) of the survey, the target species, and the types of information needed must be defined in advance. Types of surveys include (a) a basic inventory of all species, (b) an inventory of principal (target) species, and (3) a check on a specific problem or management procedure. The purpose of thesurvey is to be recorded on the completed FISH COLLECTION form to aid others in the interpretation of survey methods and results.
Careful planning, as well as execution, is essential for meeting the objective. A SURVEY PLANNING form can be used to plan surveys. The purpose of this form is to assist in review of past surveys, setting an objective for the proposed survey, and communicating this information to others. Dispose of the form after the survey report is completed.
Other forms aid in the recording and analysis of data. These allot some space to analysis and interpretation, but extensive surveys should culminate in narrative survey reports as well. Central to the forms are four tables and one figure which summarize key statistics of the fish community and its species populations. Usually, one or more of these summaries will be needed to answer your questions and diagnose management problems.
a. CATCH SUMMARY, by gear type:
| Species | ||
| Length | ||
| Avg. Wt. | ||
| Total | ||
| % | ||
| CPE | ||
| % L-A |
This table records the species taken, average length and weight, the actual catch by number and weight, the percentage contribution of the species to the sampled portion of the fish community (total % by number and by weight), an index of population abundance (CPE), and the proportion of the catch which exceeded the minimum legal size limit or was large enough to be acceptable to anglers (L-A). These key statistics generally reflect the status of the community and its species populations and are useful for detecting changes through time. At some future date, statewide averages or standards will be available for making comparisons.
b. LENGTH-FREQUENCY and LENGTH-BIOMASS,
by gear type:
'
This table, derived from a random sample of the catch, shows the size structure of the population and enables the calculation of average size and % L-A. A desirable size structure has both small and large fish, indicating that recruitment is taking place and survival and growth are adequate to produce large fish and a fishery. The optimum ratio of small to large sizes has yet to be defined for each type of gear.
The CATCH SUMMARY, LENGTH-FREQUENCY, and LENGTHBIOMASS tables are on the FISH COLLECTION form. Some space is provided on this form for analysis and interpretation. Other parameters are recorded and interpreted on the forms that follow.
c. FISH GROWTH, by gear type (form
8070):
This table records the statistics of the growth sample and compares the average length of the sample to the state average. In the analysis section of the form it is appropriate to also indicate how the growth indices compare to previous samples. Growth rate is a most useful measure of a population's well being. Slow growth commonly indicates that recruitment is not properly balanced by mortality--within the constraints of the food supply. Conversely, fast growth suggests that recruitment and overall production could be improved .
d. LENGTH-WEIGHT REGRESSION (form
):
{graph of log W versus log L}
This figure, or its equivalent equation:
is a measure of the well being (plumpness) of individuals in the population and is handy for converting length-frequency data to biomass-frequency data. Some state-average data are available now, and additional research is being conducted to develop useful standards.
e. POPULATION ESTIMATES (form ):
Species________ Estimated: No./acre____
Lb./acre____ %L-A: By No.___ By Lb. ___
More sophisticated management problems at the pop tion and community levels require absolute, rather than relative, measures , population abundance and size frequency. Mark-and-recapture methods, stratified by size groups to eliminate bias caused by size selectivity of gear, are practical in some situations-especially in wadeable streams.
While the population is being estimated,
it is usually wise to take a large number of scale samples so
that the age composition of the population can be accurately determined.
From these data, it is possible to make a good assessment of recruitment,
survival, and biological production. However, the best method
of determining survival is from age group estimates made in consecutive
years. A low rate of survival commonly signals problems of over
fishing or excessive natural mortality.
2. Procedures
It is not possible to design a single (or a few) sampling plan suitable for all fish surveys. To a considerable extent, the design of each survey must be costumed tailored to the survey objectives, species, habitats, degree of precision required, budget and time limitations, and previous experience. The following discussion of procedures is specific in routine matters (where feasible), but hopefully the more general sections will broaden the reader's understanding of sampling problems and enable him to design efficient sampling plans as the need arises.
a. Planning.-Review I-B and II-E1. The survey objectives, and the types of summaries and forms required must be established before field work begins. An important aid to every survey is a map or sketch of the lake or stream. Use it to select and record the location of sampling stations, net sets, transects, and electrofishing areas, and to note spawning areas, brush or rock shelters, land marks, and other information. The map should be stored for future reference, and as is practical and relevant, sketched on the FISH COLLECTION form or a NOTES AND REFERENCE form.
b. Forms and records.-The quality of our records reflects our degree of professionalism. In the field, use FISH COLLECTION forms to tabulate the catch and the length-frequency data (or plain waterproof data paper) and as a guide for recording the appropriate information about habitat. The LENGTH-WEIGHT FIELD DATA form is handy for taking weight data. Generally, avoid getting too complicated when recording data in the field as this increases errors and slows down the crew. For continuous recording during stream electrofishing, the formats of tables II4 and II-5 are recommended. Keep separate records of catch and effort for each gear type, collection site, and index site. In the office, as soon as possible afterwards, summarize the data, combine records for collection sites (if there is no reason to report them separately) and carefully prepare the appropriate summary forms for distribution and filing according to the instructions below and in Section IV. Store the field sheets also, if they contain potentially useful data not on the summary version.
c. Fish identification.-All fish must be identified accurately. If there is any question on identity save a sample for later examination. The I. F. R. and the University of Michigan Museum staff can provide assistance. Species which are threatened, rare, or endangered, or outside of their normal range or habitat may be of special interest to the Museum (see VI-A l l).
d. Measuring fish.-Standard units of measurement are inches and pounds (decimal).
Length. Measure total length of fish to 0.1 inch if:
(1) Fish are scale sampled for growth
(2) Fish are weighed individually or in small groups
(3) A more accurate (see below) estimate
of average size is needed (e. g., small minnows or young sport
fish)
Otherwise, measure fish to inch group.
Inch groups are defined as: 0 inch group = 0.1-0. 9 inch, 1 inch
group = 1. 0 -1 . 9 inches, etc .
Weight.
Carefully measure weights of individual fish (panfish to 0.002
pound). Very small fish may be weighed in small groups to obtain
an average weight for the inch group. Make measurements on a stable
platform, out of the wind. Extremely large catches of fish may
be estimated from bulk weights and subsample counts and weights.
e. Selection of sample sites.-Enough habitats and sites must be sampled (with appropriate types of gear) so that an experienced biologist feels confident that a representative sample has been obtained.
In surveys seeking one or a few target species, it is permissible to concentrate effort in habitats and at sites that previous experience suggests are likely to yield a representative random sample (within constraints of the gear) with respect to lengthfrequency, age-frequency, growth, or other population characteristics of interest. However, bear in mind that fish behavior is not completely predictable.
Basic inventories require a representative sample of the entire fish community and some effort must be expended in all habitats to obtain information on species diversity and fish distribution. Additional sampling effort may be expended in habitats containing (or most likely to contain) species of greatest importance. This procedure provides an experienced surveyor with the greatest amount of useful information from the least amount of effort, but invalidates a strict comparison of CPE among species.
Lakes.-Data required to complete the LIMNOLOGY form should be collected just prior to the fish survey if the lake is stratified. Use the temperature, DO, and depth information to aid in the defining of habitats and the selecting of sample sites. Other criteria useful for defining habitats are vegetation, substrate, current, cover, and morphometric features such as bays, points, inlets and outlets. Use an echo sounder to locate sample sites. Record sample site depth, temperature, and other habitat data on the FISH COLLECTION form.
Streams.-Stream surveys should be conducted within the framework that the drainage is the ultimate management unit, thus the main survey unit (see II-B2). This can be accomplished by systematically subsampling various segments (reaches) of the stream drainage. Then by summing the values obtained from the subsamples, values for the drainage as a whole can be obtained. This approach is particularly important for the assessment of fish populations and angling.
f. Index stations.-Index stations may be established to monitor seasonal or annual trends in the CPE index of abundance for a target species. An index station may be used for more than one target species, but at least 10 specimens of each species must be taken at each station, or among all stations combined, to provide useful statistics. In lakes, replicate sample each index station (e.g., at least two net nights per survey) and, for year-to-year comparisons, obtain CPE's at the same time of year with the same type of gear.
Select index stations after an understanding of habitats, and fish abundance and distribution within the lake or stream have been attained from a basic inventory. Choose some sites because large and consistent catches can be made there, others because they represent important habitats and geographic areas. Enough stations must be established, or enough supplemental sampling must be done, so that shifts in fish distribution are not misinterpreted as changes in abundance. Minimum guidelines are five index stations for lakes 10 to 100 hectares and ten stations for larger lakes.
Record the location of index stations on maps and, if feasible, on fish collection records. Check previous surveys before assigning index station numbers to avoid duplication.
Sites sampled during a survey may be assigned a temporary number, called a "Collection Site No., " rather than a permanent index number. The location of numbered collection sites is to be recorded on the FISH COLLECTION! form. Data may be summarized by collection site or index site, as indicated on the forms.
g. Selection of gear.-All types of fishing gear (including poisons) are selective by size of fish and by species. Furthermore, their efficiency varies according to habitat.
To inventory a target species, the most effective gear should be selected. For comparison with an earlier survey, use the same gear as before .
For a basic inventory of the fish community, the sampling gear should be adequate and diverse enough to sample all habitat types and all species in rough proportion to their abundance. Basic lake surveys require the use of gill nets, trap nets or fyke nets, plus seines or 220-volt AC electrofishing equipment. In shallow lakes (less than 30 feet deep), allot more effort to trap netting than to gill netting; in deep lakes, do more gill netting than trap netting.
In wadable streams, the best gear for sampling fish is the 220volt DC stream shocker.
Non-wadable streams are difficult to sample. Boom shockers with 220-volt AC or 220-volt DC are usually the best types of gear. In sluggish current, fyke nets or seines maybe useful. Rotenone may be used to sample river populations (e . g., Grand River in 1978) . The fish are collected in a blocking seine at the lower end of the sample areas. The rotenone is detoxified with potassium permanganate as it leaves the sample areas.
h. Duration and effort.-A
survey should continue long enough, and be intensive enough, to
obtain a representative sample of all important species. Usually
this means a minimum of 30 fish of each of the species. This goal
may not be feasible if the fish prove to be difficult to catch
(e.g., mid-summer netting in lakes). Netting in lakes should
extend over two or more nights. The following table may be used
as a guide for planning the amount of netting (trap + fyke + gill)
required for an adequate sample:
i. Catch per effort (CPE).-Catch per effort is a useful index to fish abundance, especially for monitoring changes in a species at index stations. Standardized gear and effort are prerequisite. For all fish surveys catch and effort are to be recorded for each gear type, and corresponding CPE's are to be calculated on the FISH COLLECTION form unless the collector notes why the CPE statistic would not be representative. Possible reasons for a nonrepresentative statistic include faulty gear, incomplete records of catch, or nets not being set overnight. Catch per effort is expressed as both number and weight caught per unit of effort. Catch per effort information should be part of final reports and should be used for comparisons with past surveys (Table II-2). It should be understood that CPE is a highly variable statistic and that only major increases or decreases or clear trends through time should be interpreted as reflecting real changes in fish abundance.
Selectivity of gear makes comparisons of CPE across species difficult. Rather, the relative abundance of species in the community should be expressed on a rank basis (rare, sparse, common, or abundant).
{Table II-2}
Table II-3.-Standard units of effort
for CPE (Part A); and comparison of three types of CPE for trap,
fyke, and gill netting (Part B).
| Gear | Standard units |
| Trap or fyke net )
Inland experimental gill net) Great Lakes gill net ) | Catch per net lift (with overnight sets) |
| Large seine | Catch per acre seined |
| Minnow seine | Catch per haul |
| Toxicant sampling | Catch per acre of area sampled |
| Trawl | Catch per 5-minute unit of "actual fishing time" or catch per acre |
| Visual observations | Adjust as appropriate |
| Angling | Catch per hour |
| Set hooks | Catch per set hook per lift |
| Electrofishing | |
| Lakes and non-wadable streams | Catch per hour of actual fishing time
(15 minutes minimum effort) |
| Wadable streams | Catch per mile or catch per acre |
| etc. | ||||
a "Net lifts" are the standard divisor for trap, fyke, and gill netting CPE computations on the FISH COLLECTION form (R8058). A net lift is defined as a set over one or more nights (i. e., excludes sets not made overnight).
b "Net nights" are an optional, more precise, unit of CPE. Record the number of net nights in the space provided on the front of the FISH COLLECTION form for possible use. A net night is defined as a lnight set.
c "Nights
of netting" is another optional measure of CPE for use in
reports or analyses. Nights of netting is defined as the total
number of nights a net was fished, irrespective of the number
of lifts.
More precise measures of fish community structure require actual population estimates of each species, or CPE's adjusted for gear selectivity.
Table II-3 presents units of effort required to calculate CPE for various types of gear.
j. Length-weight relationship.-Individual lengths and weights of important species should be obtained during inventories so that length weight regressions can be computed. Use the regressions to determine relative plumpness, and (see II-Eh) to expand lengthfrequency data to length-biomass data and total biomass of the catch.
Obtain the individual lengths and weights on a sample of about 10 fish per inch group per species. For small fish which are difficult to weigh individually, weigh all 10 fish together to obtain an average. Weigh panfish to 0.002 pound (1 gram), if possible. Take the weights carefully, on stable footing, out of the wind. Record lengths and weights on scale envelopes, if scale samples are being taken, or on LENGTH WEIGHT FIELD DATA forms. Later, transfer data from the scale envelopes to SCALE SAMPLE ANALYSIS forms. Computer analysis of these forms is available, saving step 1 below:
l. Calculate: log W = log c + n log L
or plot W and L on log-log graph
paper:
{graph of W versus L}
2. Fill out the LENGTH-WEIGHT REGRESSION form. Evaluate relative plumpness by comparing the regression slopes (n), or the displacement of the lines on a graph, to prior samples. In the example graphed above, the fish are now heavier at the same length than they were in 1975. State standards (VI-A12) may also be used for comparison. Keep seasonal changes in mind (e.g., spawning) when making comparisons.
k. Length-frequency.-taken for length-frequency analysis must faithfully reflect the size structure of the catch and, within the limits of gear selectivity, should reflect the true size structure of the population. The measured fish must be selected randomly or systematically. Generally for management surveys, the first 200 fish caught of each species should be measured to inch group, but very large catches should be subsampled so that a variety of sample sites and dates are represented. Lesser numbers may be measured if the range in fish size is unusually small. Avoid subsampling from catches held in tubs or other containers, as the subsample will almost certainly be biased. It is better to measure all the fish caught in every other net rather than to pool the total catch in a tub and try to randomly pick out half of the fish. Also, do not select specimens on the basis of size with one exception: the largest or the smallest specimen may be added to the length-frequency table if it was not included in the 200 already sampled. This allows the full range in size within the catch to be conveniently recorded.
The length-frequency of the sample is to be reported on the FISH COLLECTION form. A rough draft of the form may be used for tabulating data in the field.
l. Length-biomass and total biomass.-Biomass of fish is a better measure of productivity and of community structure than numbers of fish. On the population level, a length-biomass table (FISH COLLECTION and POPULATION ESTIMATE forms) indicates at which size a species has accumulated its greatest net production--after that size the population loses more biomass to mortality than it gains from growth. On the community level, expressing species composition as a percentage by weight compensates for the large differences in the average lengths of the species.
Obtain length-biomass data for the random sample of fish used for the length-frequency table either directly by weighing all the fish in each inch group, or indirectly (usually the most practical under field conditions) by multiplying the number of fish caught per inch group by an average weight for fish in each group. For the indirect method, obtain an average weight for each inch group by one of the following:
1. Adding the empirical weights taken for the length-weight relationship and dividing by the number of fish weighed (LENGTHWEIGHT FIELD DATA form);
2. Calculating from the length-weight regression equation (or simply reading from the graph), by assuming the average length of fish in the inch group was the midpoint (e . g., 6.5, 7.5, etc . );
3. Using the state average length-weight
tables (VI -A 12).
After the length-biomass table has
been completed, calculate for each species an average weight and
the total pounds caught, then the other statistics required for
completion of the forms.
Example: 80 perch (plus other species) were taken in two experimental gill nets. Of these, 68 were measured to inch group (shown) and 48 were measured to 0.1 inch and 0.002 lb. (not shown, recorded on a LENGH-WEIGHT DATA form). Average weights for the inch groups were: 5-inch, 0.060; 6-inch, 0. 101; 7-inch, 0.149; 8-inch, 0.230; 9-inch, 0.312. Biomass estimates were obtained by multiplying each average weight by the number of perch in each group (e. g., for 5-inch group: 0.060 X 12 = 0.72 lb.). The table was then completed:
Avg. Wt. = 12.08 lb. /68 = 0.178
Total Lb. = 0.178 lb. X 80 = 14.24
%L-A No.= 41/68 = 60.3
%L-ALb.=9.841b./12.081b. = 81.4
CPE No. = 80/2 = 40
CPE Lb. = 14.24/2 = 7.12
Note the rounding off in the table.
{table saved as TII-p38.doc}
m. Average length and weight.-Designated as "size, no. " and "size, lb. " on the FISH COLLECTION form. Calculate from a random or systematic sample, usually from the length-frequency and biomass-frequency tables.
The best estimate of the average length of small samples of fish is the simple average of individual measurements which were made to 0.1 inch. A satisfactory estimate of average length may be computed from a large length-frequency sample by a weighted formula which assumes that the 0-inch group fish average 0. 5 inch long, the l-inch group fish average 1 5 inches long, etc.
Each median length is multiplied
by the number of fish in the inch group, the products summed,
then divided by the total number of fish. Below is calculated
the average length of the 68 perch in the preceding example (II-E2n).
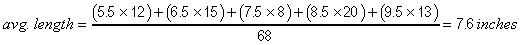
The best estimate of average weight is obtained by dividing the total biomass in the biomass-frequency table by the number of fish in the length frequency table. See the example in II-E21. Alternatively, divide the empirical weight of the total catch by the total number of fish.
n. Growth.-Samples taken for age and growth analysis should fairly represent the ages and growth rates within a species population. Subsamples may be taken from the catch systematically (e. g., every other fish), randomly, or on a stratified-random basis (e. g., 15 randomly selected samples from within each inch group).
The stratified-random method is best when the catch is large, when a length-frequency sample is also taken, and when age groups cannot be clearly identified in advance on the basis of length or stocking records. For most management surveys of growth a sample of 10-15 fish per inch group is adequate. That will usually result in a sample of at least 15 per age group. For more intensive studies of growth and age composition (as in conjunction with population estimates), a sample of at least 30 fish per inch group should be taken (see II-Ep). Appendix VI-A1 discusses general aspects of sample size in greater detail. It is better to take too many samples (not all of them need be examined) than too few.
The techniques of scale sampling, aging, and back calculation are discussed in VI-A4. There are two methods for calculating the average length of an age group of fish. If the sample was taken systematically or randomly, then a simple average of the data is appropriate. However, if a stratified subsample was taken, a simple average gives an overestimate in most instances and it is better to calculate a weighted average length with the aid of length-frequency information, as illustrated in VI-A 10. The method used for calculating average length is to be recorded in the space provided on the Fish GROWTH form.
Statewide growth averages and computed growth indices (see VI-A4) may be used as standards for comparing the growth of one population with others. However, in judging if the observed growth is satisfactory or meets expectations, other factors such as the productivity of the water and the type of fish population should be considered. The state averages have been broken down into four time periods per age so that more meaningful comparisons can be made between samples taken at different times of the year. For example, age-III largemouth bass "should" average about 9.4 inches in January-May (prior to that growing season) and about 11.6 inches in October-December (after that growing season). If the observed length of age-III bass in Example Lake was 10.4 inches in May 1960 (growth index = +1.0), and 10.6 inches in November 1970 (growth index = -1.0), then it is clear that bass growth has declined (2.0 inches).
o. Population estimates.-Estimates of the actual density of fish may be obtained by (1) a complete census of the entire water body or a portion of it, e.g., draining or poisoning followed by complete recovery; (2) catch per unit of effort adjusted for gear efficiency, e. g., catch per area seined, trawled, or electrofished; or (3) by one of the variations of the mark-andrecapture technique. Because complete recovery of fish is rarely possible and the efficiency of gear is difficult to assess, the mark-and-recapture method is usually the best.
Markand-recapture data of the Petersen type (e. g., trout in streams) may be submitted for machine computation by entering the raw data on the left-hand side of the POPULATION ESTIMATE form. After the estimates are computed, the rest of the table is to be completed and distributed. Estimates derived from other types of formulas (e. g., Schnabel) should be summarized on the same form, if possible.
For details on mark-and-recaptured methodology refer to VI-A2 (streams) and VI-A3 (lakes) and to standard references such as W. E. Ricker's (1975) "Computation and Interpretation of Biological Statistics of Fish Populations, " Bulletin 191, Department of the Environment, Fisheries and Marine Service, Ottawa, Canada. Several points about mark-and-recapture estimates merit is:
1. It is usually wise to collect scale samples during population estimates so that age-frequency and survival can be studied concurrently (see II-Ele and II-E2k).
2. They are highly recommended for management surveys of wadable streams because much better information is obtained for only about twice as much effort as a once-through electrofishing survey. The Bailey modification of the Petersen formula is the most appropriate. See II-B2c for specifics on length of stations. 3. They are more accurate (and sometimes less work) than a complete census of chemically treated waters. Mark native fish prior to the treatment and then examine a large sample of the dead fish to obtain the ratio of marked to unmarked fish.
4. They must be stratified by species and size, then summed, to compensate for gear (and people) selectivity. If possible, use one type of gear to catch fish for marking, another type of gear for the recapture sample.
5. The most critical underlying assumption is that marked fish have the same probability of recapture as any other fish in the population in the recapture sample.
6. Care must be taken to sample all parts of the study area. For example, use extra long electrodes to sample trout living in deep pools of streams. Alternatively, conduct the estimates when the fish are mixing freely and are equally vulnerable to capture. Such mixing occurs on the shoals of lakes during spring and fall.
7. Valid estimates can be obtained even after a long lapse of time between marking and recovery (e. g., fall to spring), provided:
a. Marks are not "lost".
b. Marked and unmarked fish have the same survival rate.
c. Fish are not subtracted or added to the population because of movement or recruitment.
8. Concentrate sampling effort on the target species. For example, in electrofishing wadable trout streams, concentrate on catching trout and do not attempt to make quantitative catches of other species (muddlers, minnows, suckers, darters, etc. ) at the same time, because trout catches (and estimates) will suffer. Simply note if other species are abundant, common, or rare. If better population data are needed for these non-target species, then conduct a estimate (see Ricker [1975] for methods) in a short section of the stream.
Example.-Brook trout in a stream were sampled with a 220-volt DC stream shocker. They were marked by clipping the top lobe of the caudal fin. Scale samples were taken. Field data from the marking and recovery runs are shown in Tables II-4 and II-5.
In the office, the data were tallied, and population estimates were made by inch group using the Bailey modification of the Petersen formula (Table II-6, see also lII-A2). It is better than the simple Petersen formula when sample sizes are small, as is typically the case. Direct estimates of the 1-, 12-, 13- and 14inch groups could not be made reliably because fewer than three recaptures were made. Therefore, data for the 1- and 2-inch fish were combined and a single estimate calculated. For the large trout, it is apparent that nearly 100% of them were caught and the best estimate is simply the sum of the catch. Alternatively, we could have calculated the ratio of number of marked fish to the population estimate for every other size group, plotted these ratios versus size groups, fit a line or a curve to these points, read the ratio off the graph for the size group with insufficient data, then expanded the number of fish marked by this ratio. Population estimates should be expressed in fish per acre, fish per mile, or fish per unit of discharge (Table II-6). Biomass of the population should also be computed if the length-weight relationship is known.
Using the age composition of the scale sample collection, the estimates by inch groups were converted to partial estimates by inch groups and age groups as shown in Table II-6. For example, of the 317 4-inch trout per hectare, 41. 7% were age O (132 fish) and 58. 3% were age I (185 fish). The total estimate for the age group is then the sum of these partials.
From the estimates by age groups just derived, the apparent survival rate of fish in the population was estimated. The survival rate is equal to the percentage of fish surviving to the next older age class, if recruitment is exactly the same each year. These rates were 32. 9% for age O-I, 48. 4% for age I-II, 10. 2% for age II-III, 13. 3% for age III-IV. A plot of the abundance of each age class on semi-log paper gives a graphic picture of survival rate (Fig. II5). Note the term "apparent" survival rate. This is because one cannot be sure whether decreases in numbers at a particular station are due to mortality or to movement out of the station which is why it is best to look at the population on a drainage basis.
Good population estimates at all the sample stations of the drainage provide the means to estimate the population for the entire drainage. To do this, assume that the sampling station (located near the center of the drainage zone) is representative of the zone as a whole. Then calculate the population within each drainage zone by multiplying the population per acre found within the station, times the number of acres in the zone. To arrive at the population of the drainage, the populations of all zones are summed.
From the data on numbers of fish in each age and size class, a weighted estimate of growth rate was made (Table II-6). For example: the number of age-O (fall fingerlings) in each size class was multiplied by the mid-point of that size class to arrive at total inches. This was done for each size class where age-O fish were represented. Total inches were summed and divided by the total estimated number of age-O fish to get the average size of age-O fish. The procedure was repeated for each age group. Another example is provided in VI-A10. This method reduces most sampling bias but has limitations in that it requires rather extensive data. A graphic picture of the growth rate is in Fig. II-6.
p. Age-frequency and survival.-Age-frequency information may be used to simply identify weak and strong year classes or, more rigorously, to compute survival rates. Routine management surveys of growth often collect adequate information to rank the relative strength of year classes (note that stratified subsamples must be weighted as in VI-A10), however careful planning and larger samples are needed for reliable estimates of survival.
For most purposes, studies of survival should be made in conjunction with population estimates. Obtain at least 30 scale samples per inch group. Methodology is presented in detail in IIE30 and VI-A10. The computations are to be summarized on the POPULATION ESTIMATES form.
Survival may also be estimated from simple "catch curves" by substituting catch frequencies for mark-and-recapture population estimates. See textbooks for discussions of methods and limitations. This method is not as reliable because catch frequencies are biased by gear selectivity.
Estimates of annual survival rates based on age frequencies taken on one date (whether based on mark-and-recapture estimates or simple catch curves) are subject to errors caused by uneven year class strength. Therefore, it is best to estimate the population in two consecutive years and compute the survival of each year class directly as the number alive in year 2 divided by the number alive in year 1.
For an example of the computation of survival rate see the trout data in the preceding section (II-I 30).
q. Production.-Production, the result of the interaction between growth and mortality, is useful for computing maximum sustainable yields and in selecting the most appropriate fishing regulations. It is narrowly defined as the total elaboration of fish tissue during any time interval (usually a year), including individuals that do not survive to the end of the interval. It is obtained by multiplying the instantaneous rate of increase in individual weight by the average biomass of the population during the time interval. Thus, the basic data required are growth, survival, and the biomass of the population. Production can be determined by means of a graph (Allen method), equation, or computational table. See references such as: W. E. Ricker (ed. ) 1968. Methods for assessment of fish production in fresh waters. IBP Handbook No. 3. Blackwell Scientific Publ., Oxford and Edinburgh.
r. Natural history observations.-Record
field observations on fish movements, spawning, disease, parasites,
etc. on FISH COLLECTION or NOTES AND REFERENCES forms. These observations
are important. If a number of fish have disease or unusual features,
make accurate observations and count and weigh them. Save some
specimens on ice for later examination by a pathologist or other
specialist.
Observations on the fishery should be recorded on the FISH COLLECTION or NOTES AND REFERENCES form. Recorded observations should usually be limited to fish observed; however, local reports of success or complaints may be recorded if the biologist feels the account is reliable.
Creel census should be used to document the success of significant management programs. Creel census methods are contained in VI-A9. Assistance in conducting a creel census is also available at the Institute for Fisheries Research in Ann Arbor.